The Buzz on Herbalife
The Buzz on Herbalife
Blog Article
The Greatest Guide To Herbalife
Table of ContentsSome Known Facts About Herbalife.Herbalife - The FactsThe Only Guide to Herbalife3 Simple Techniques For Herbalife
Herbal medicines are usually offered as food supplements, yet a typical governing framework does not exist in various nations. Therefore, info on clinical signs for their usage, efficacy, and security are influenced by the traditional experience readily available in each location. A quick overview of the legislation in United States, copyright, and Europe is given up this area, and could be made use of to direct the lawful elements of the herbal medication industry in other nations.Nutritional supplements do not need authorization from the Food and Drug Management (FDA) prior to they are marketed (FDA 2010. herbalife protein powder. Under DSHEA, natural medications, which are categorized as dietary supplements, are assumed risk-free, and the FDA does not have the authority to require them to be authorized for safety and security and efficiency prior to they enter the market, which holds true for drugs
A dietary supplement maker or representative of a supplement with a "brand-new dietary ingredient," that is, an active ingredient that was not marketed in the United States before October 1994, may be required to go with premarket testimonial for security data and various other details. All domestic and international firms that produce bundle tags or hold nutritional supplements must follow the FDA's current good production practice (GMP) regulations, which describe procedures for making certain the quality of supplements intended for sale (FDA 2010; Gao 2010).

The Ultimate Guide To Herbalife
In order to be granted a permit, detailed details on the medical components, resource, potency, nonmedicinal active ingredients, and advised usage needs to be equipped. As soon as an item has actually been given a license, it will certainly birth the certificate number and follow typical labeling demands to make sure that consumers can make informed choices.
In enhancement, GMPs need to be employed to ensure product safety and security and top quality. https://www.cybo.com/ZA-biz/herbal-product-life. This calls for that proper criteria and practices regarding the manufacture, storage space, dealing with, and distribution of natural health items be met. The GMPs are created to be end result based, guaranteeing secure and premium items, while giving the flexibility to implement quality assurance systems ideal to the product and company
In Europe, the European Directive 2004/24/EC released in 2004 by the European Parliament and by the Council of Europe supplies the guidelines for using herbal medicines (Calapai 2008 (herbalife pricing). The directive develops that natural medicines released on the marketplace requirement authorization by the nationwide regulative authorities of each European country which these items have to have a recognized degree of security and efficacy (Calapai 2008
With regard to the production of these items and their quality, items must accomplish the exact same requirements as applications for a marketing permission. Information is based upon the availability of modern sciencebased public essays in the European Pharmacopeia and their equivalents created by the pharmaceutical industry. The criteria advanced allow not only to specify the top quality of items yet likewise to eliminate hazardous substances, debauchment, and contamination.
Rumored Buzz on Herbalife
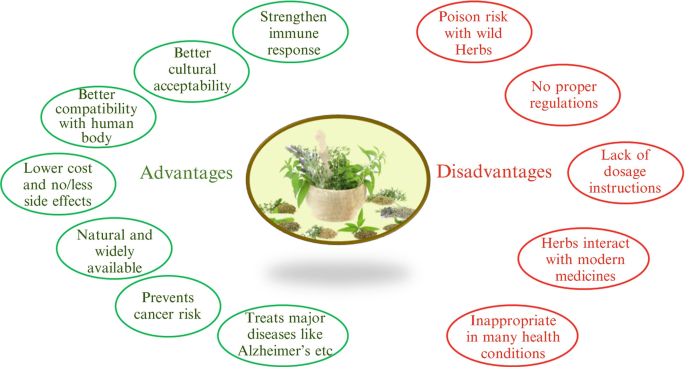
To isolate each active component from each natural herb would be tremendously time-consuming at a high cost, making it not affordable for manufacturers (Richter 2003. An additional trouble is that regardless of the popularity of herb nutritional and organic supplements, some natural products on the market are most likely to be of poor quality and suspect effectiveness, even if the natural herb has actually been shown to have an effect in controlled research studies making use of premium product
Natural herbs may well have unfavorable side results, there are no collection "doses," and herbdrug or herbherb interactions are possible. A significant theoretical advantage of botanicals over standard single-component medicines is the presence of several active substances that with each other can offer a potentiating effect that might not be possible by any type of single substance.

Not known Incorrect Statements About Herbalife
The top quality control of herbal medications has a straight influence on their security and efficiency (Ernst, Schmidt, and Bigger 2005; Ribnicky et al. 2008. However, there is little information on the composition and high quality of the majority of organic medications not just due to lack of ample plans or government needs but likewise as a result of a lack of sufficient or accepted research study technique for examining conventional medications (THAT 2001; Kantor 2009. To isolate each energetic ingredient from each natural herb would certainly be immensely taxing at a high expense, making it not economical for producers (Richter 2003. Another trouble is that regardless of the popularity of botanical nutritional and organic supplements, some natural items on the marketplace are most likely to be of poor quality and suspect efficacy, also if the herb has actually been shown to have a result in regulated researches using premium item
Natural herbs might well have undesirable side effects, there are no collection "doses," and herbdrug or herbherb communications are possible. A significant theoretical advantage of botanicals over standard single-component medicines is the presence of multiple energetic substances that together can provide a potentiating result that may not be achievable by any type of solitary compound.
Compounds that are identified by activity-guided fractionation has to be evaluated in ideal animal designs to validate in vivo activity. Preferably, the composition of the complete botanical extract need to be standard and without any prospective dangers, and plants ought to be expanded especially for the production of botanical extracts under regulated conditions and originate from an identified and consistent genetic resource with a taxonomic record of the genus, types, and cultivar or other added identifiers.
Report this page